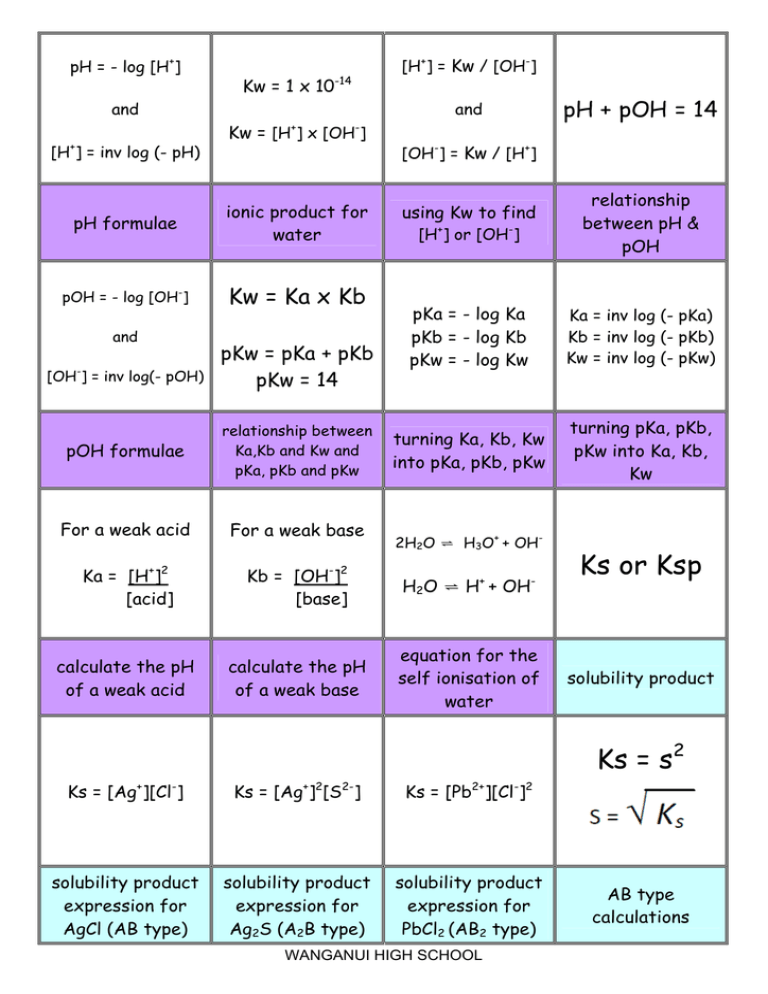
AutoIonization of Water, Ion Product Constant - Kw, Calculating H3O+, OH-, and pH Using Ice Tables - YouTube
The ionization constant for water (Kw) is 9.311 × 10−14 at 60 °C. What is the [H3O+], [OH−], pH, and pOH for pure water at 60 °C? Thanks. - TopScience - Quora

pH from Base concentration and Ionic Product of Water calculation Workthrough - A2 Chemistry - YouTube

pH, pOH, H3O+, OH-, Kw, Ka, Kb, pKa, and pKb Basic Calculations -Acids and Bases Chemistry Problems - YouTube
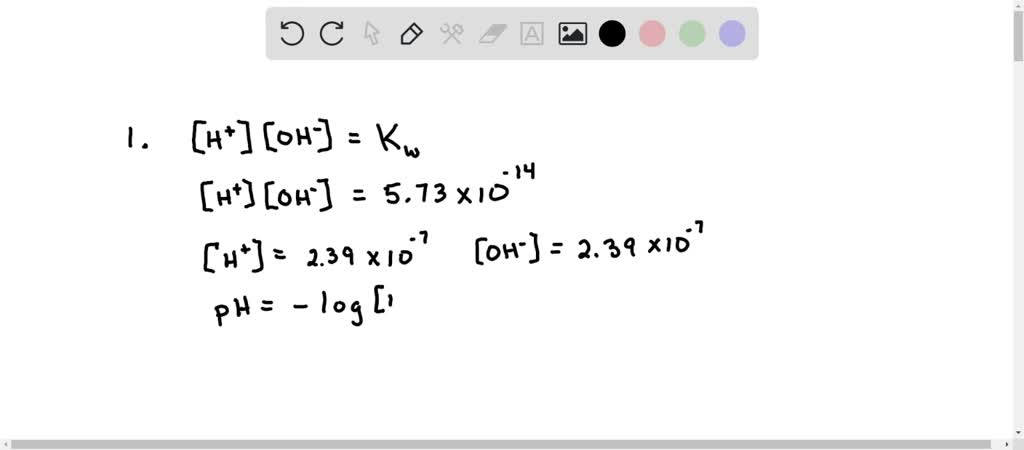
SOLVED: At 323 K, the value for Kw changes and is found to be 5.73 x 10 -14 . (i) Calculate the pH of water at this new, higher temperature. (1) (ii)
![SOLVED: At 50°C, the value of Kw is 5.47 x 10^-14. a) Calculate the [H+] and [OH-] in pure water at 50°C. [H+] M [OH-] M b) What is the pH of SOLVED: At 50°C, the value of Kw is 5.47 x 10^-14. a) Calculate the [H+] and [OH-] in pure water at 50°C. [H+] M [OH-] M b) What is the pH of](https://cdn.numerade.com/ask_previews/ed96ab60-48fb-4213-8fd0-3c129172f46d_large.jpg)
SOLVED: At 50°C, the value of Kw is 5.47 x 10^-14. a) Calculate the [H+] and [OH-] in pure water at 50°C. [H+] M [OH-] M b) What is the pH of
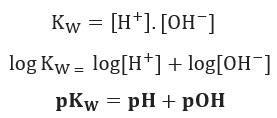
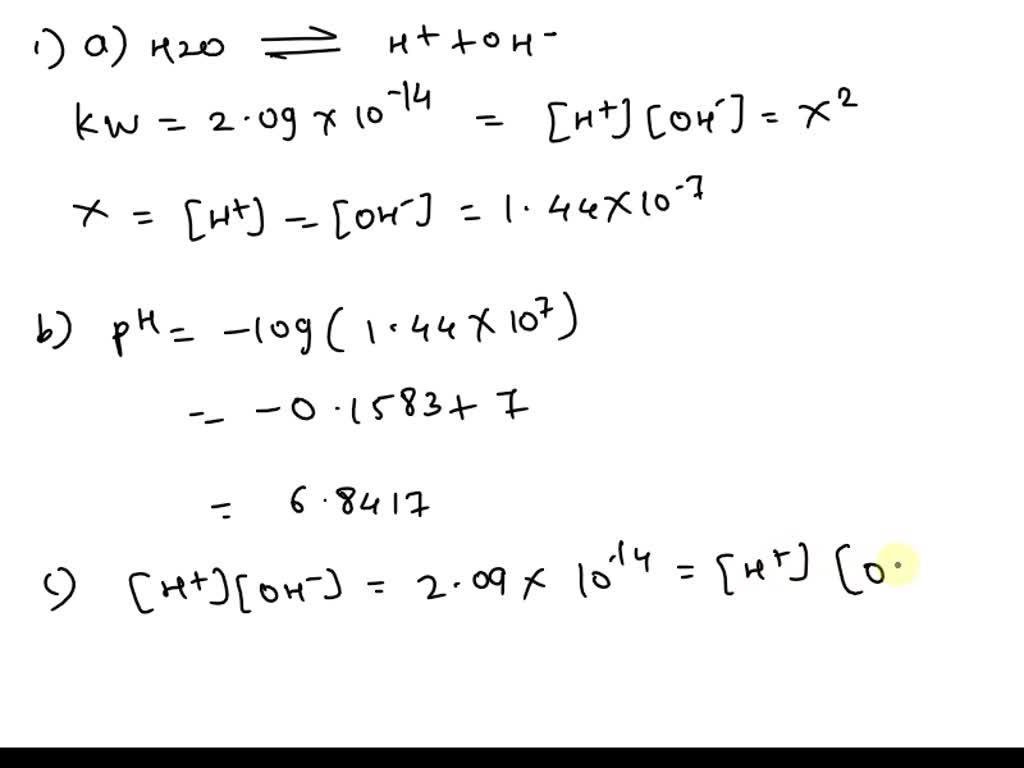
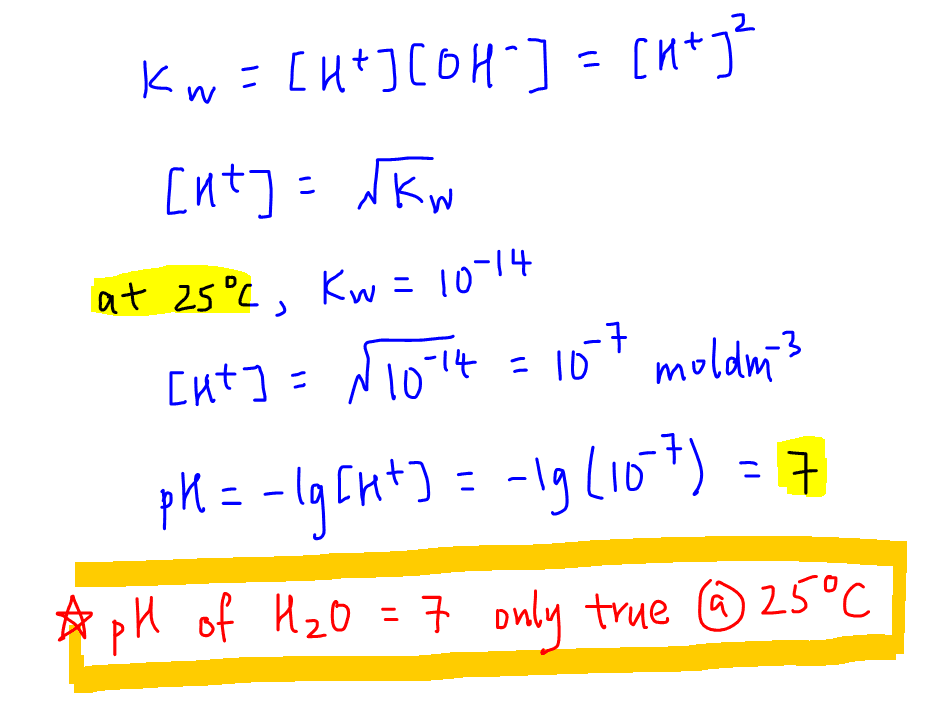
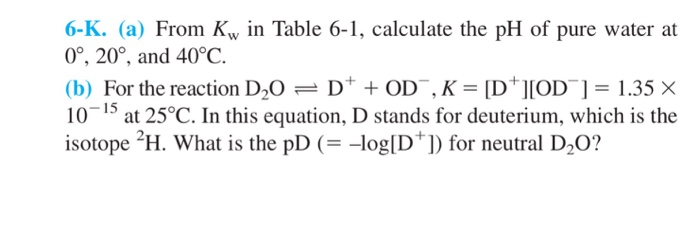
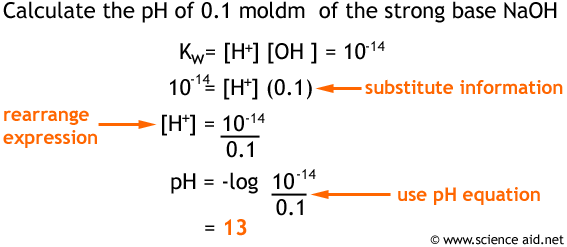

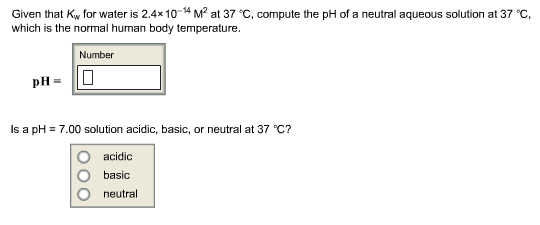
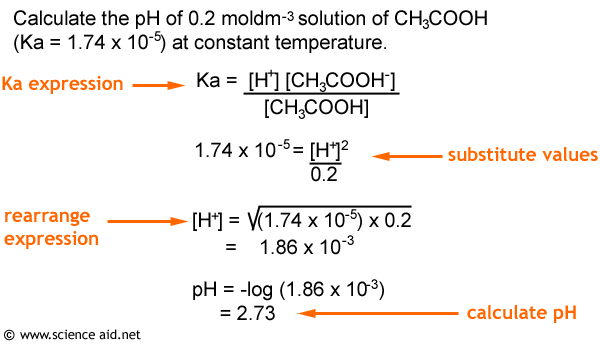
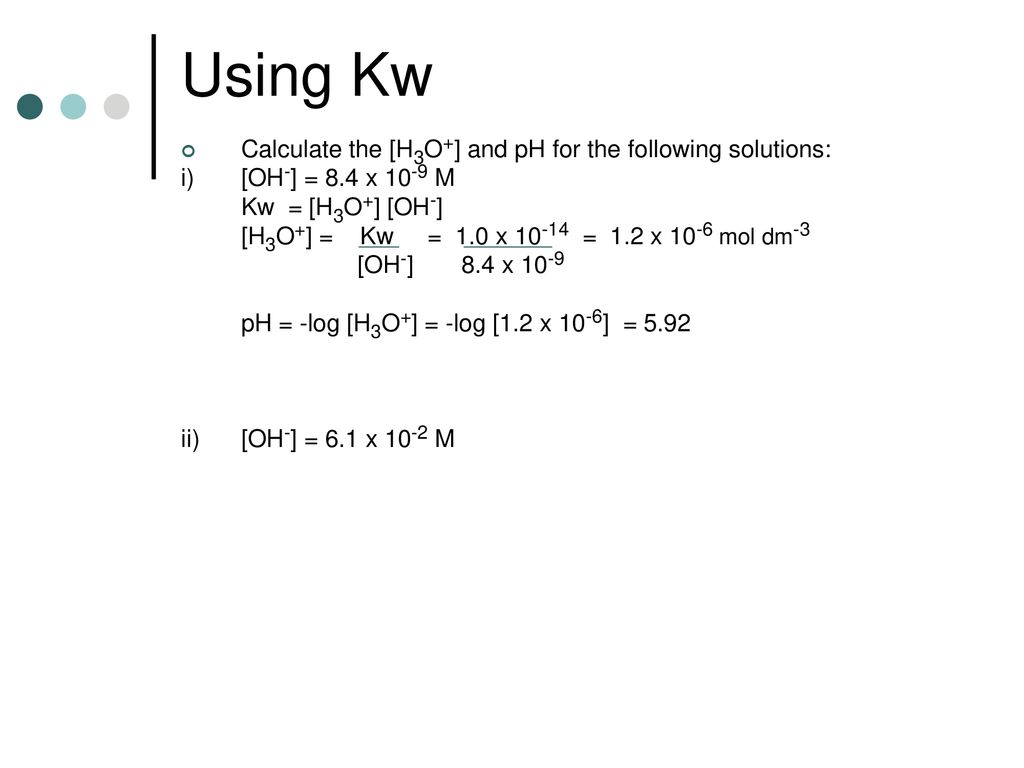
![Calculating [H₃O⁺] and pH (worked examples) (video) | Khan Academy Calculating [H₃O⁺] and pH (worked examples) (video) | Khan Academy](https://cdn.kastatic.org/ka_thumbnails_cache/e67920d2-30a0-40ef-bcfb-a7761c9673f6_1280_720_base.png)




![Acids and Bases Part 4: Kw and Calculation of [H+] and [OH-] - YouTube Acids and Bases Part 4: Kw and Calculation of [H+] and [OH-] - YouTube](https://i.ytimg.com/vi/IvP_PxetNUw/maxresdefault.jpg)